An introduction to Steel Heat Treatment
Consider the description of a steel in a metal merchant's brochure. It might well be as follows; "A high quality alloy steel specification usually supplied as a high tensile steel. This grade offers good ductility and shock resisting properties combined with resistance to wear. At low temperatures it has reasonably good impact properties."
The key attributes of a particular steel describe its physical and mechanical properties, and these are seldom the aesthetic. The physical characteristics of steel, like Bendability, Hardness, Ductility, Toughness, which make it useful across a wide variety of applications can be quantified using various more precise measures such as Tensile Strength, Yield Stress, Elongation, Hardness. These are all mechanical properties and they are governed by the microstructures within the steel itself.
We can design what kind of microstructure the finished steel has and we do this by:
- firstly controlling the chemical composition of the steel (% Carbon, Manganese, Silicon etc), as well as
- the actual types of microstructure present in the steel, which we do through various heat treatment (and cooling) processes.
Heat treatment is really the application of well understood processes to carbon steel to control the microstructures present in the material and therefore the mechanical properties that the material will exhibit.
The Carbon Steel Phase Diagram
The accompanying diagram, explains the relationship between temperature, cooling and micro structures in steel.

At temperatures below the A1 line, steel is made up of ferrite and pearlite; they have their own structure. When the steel is heated to a temperature above the A1 line, a new phase called austenite begins to be formed and is present in the steel with the original ferrite. Now, if the steel is heated to above the A3 temperature all the ferrite will transform to austenite and the structure will be fully austenitic.
What happens when we reverse this, ie instead of heating the steel we cool it? We might expect the austenite to transform back to the ferrite but we find that the rate at which this cooling takes place has a profound influence on its room temperature microstructure and therefore its mechanical properties.
If the steel is cooled rapidly, the carbon atoms are unable to diffuse through the steel and become trapped causing distortions of the atomic lattice and instead of ferrite a new structure called martensite is formed. This has the property of hardness and/or strength.
If we have slower cooling rates, other structures with different mechanical properties form. So heat treatment is the initial heating and then most importantly, the controlled cooling at required rates which allow us to obtain the required microstructure in carbon steel for the properties we need. We simply control, through the cooling rate, the formation of the microstructure in the steel that governs the mechanical properties of the finished steel, i.e. its strength, toughness and hardness.
The red curves in the graphic above represent different cooling rates (related to quenching intensity H) when cooled from the upper critical (A3) temperature. V1 produces martensite. V2 has pearlite mixed with martensite, V3 produces bainite, along with pearlite and matensite.
Quenching
The precise tempering temperature and time depend on the desired properties and the purpose for which the steel is to be used. Now here’s the hassle, Heat treatment temperatures, including rate of heating, cooling and soaking times will vary due to factors such as the shape and size of each steel component, apart from the chemical composition which we already know about

In a production environment, there are plenty of tables available that provide rule of thumb for the temperature soak time, ie the amount of time required above a certain temperature or following a certain temp path, so that all the ferrite will have melted to form the initial austentite.
But the heating/soak phase is far easier to get right than the cooling process.
Jominy hardenability curve
The term Hardness is not to be confused with hardenability. Hardenability, is the ability of the steel to achieve a hardness value at a particular depth beneath the surface. Recall that the rate of cooling is critical, and this is obviously dependent of the material from the cooling surface.
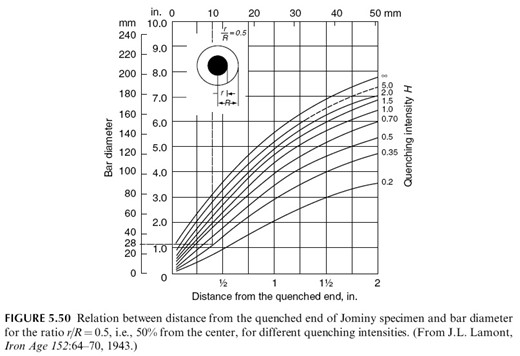
We use this diagram to determine the maximum diameter of the theoretical bar (ie the OD of our component) that will achieve a particular hardness at a specified location on the cross section when quenched under specified conditions. Here we have the diagram specifically for SAE 4140 for r /R = 0.5
This tells us for example that for hardness of 55 HRC at a Jominy distance of 10 mm, then the maximum diameter of the bar that will achieve this hardness at half-radius when quenched in oil with H=0.35 will be 28 mm. (taking the vertical line at a Jominy distance of 10 mm to the intersection with the curve for H=0.35, giving the value of 28 mm on the ordinate.)
So we can see that the geometry and dimensions of what we are working on must influence the character of the cooling curves. to achieve a through hardening of bulky products or full martensitic hardening to the core of a product, one has to provide the critical hardening rate along the entire cross section of the product. Note that hardenability depends on the steel composition, specifically on the carbon content.
Tempering
Let's assume we have conducted this first phase of heat treatment, the heat-soak then quenching.

We will now have a component that is hard and strong, but yet which is also brittle. Brittle means it cannot be plastically deformed and although strong is unable to resist impact loads, is will be extremely sensitive to stress concentrations. Some of the hardness and strength must be sacrificed to obtain suitable ductility and toughness. This is done by tempering the steel a process that reduces the brittleness of a steel without significantly lowering its hardness and strength.
Managing what's iportant
Tempering temperature has a much more dominant effect on tempered hardness than tempering time

The data in the Figure, specific in this case to SAE 4140, but illustrates that for each material there are empirical data from which to derive heat treatment parameters.
In this instance, a soak at the tempering temperature recommendation of 170°C for 2 hours per 25mm of ruling section, then allowing to cool in air .

The results from tempering depend on the time of treatment. The longer the time of treatment (at a given tempering temperature) the better are the results. It is recommended that for getting satisfactory results at least one hour be allowed at any tempering temperature. Some tempering operations consume several hours. For chromium steels, it is imperative that quenching be accompanied by subsequent tempering.
Key Points to Realise
These are considerations to keep in mind when working with Spring Steel:
- Even the best Spring Steel, badly or incorrectly heat treated, will not perform as desired.
- Before deciding on a grade, make sure that the mechanical properties have been agreed
- Take time with your metallurgist and discuss the whole heat treatment quenching and tempering cycles with them
- The Hardenability of a metal is as important, if not more so, than the "hardness" of it
- Demand more than just the material Mill Certs, and a Hardness Test, ask for the Heat Treatment cycles and have them qualified.